Abstract
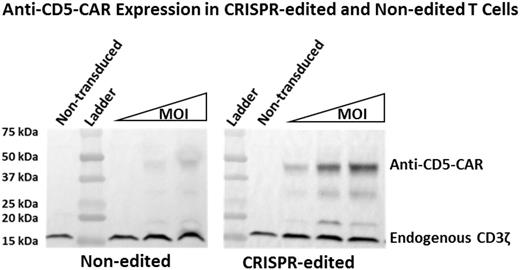
Remission reinduction poses a great challenge in treating relapsed T-cell malignancies. These patients have poor outcomes when treated with chemotherapy alone. Treatment of relapsed B-cell malignancies using chimeric antigen receptor (CAR) T-cell therapy has had great success. However, the same approach will be difficult to apply to the treatment of T-cell malignancies due to the lack of T-lymphoblast specific antigens. Therefore, T cells modified with anti-T cell CARs target themselves, potentially resulting in fratricide of CAR T cells and killing of normal T cells. We have been investigating mechanistic approaches to evade fratricide-based limitations of CAR therapy for T-cell leukemias. Since the majority of T-cell leukemias express CD5, we hypothesized that if CD5 expression was knocked down/out in effector T cells, the issue of fratricide would be eliminated. We used CRISPR/Cas9 genome editing to knock out CD5 expression in CD5-positive T cells, resulting in CD5-negative T cells that were used as CAR-expressing effector cells. In order to target CD5, CARs were generated using two structurally different antigen recognition sequences - a traditional immunoglobulin-based single chain variable fragment (scFv) and a lamprey-derived variable lymphocyte receptor (VLR). We have previously shown this VLR can be used as an alternative for CAR-based antigen recognition. Here, we compare non-CRISPR T cells to CD5-edited T cells when modified with either a CD5-VLR-CAR or CD5-scFv-CAR. Our in vitro studies show both CARs yield comparable T-cell activation in non-CRISPR T cells and that CD5-edited CAR-modified T cells have a substantial reduction in self-activation. Furthermore, CD5-edited CAR-modified cells become activated in the presence of CD5-positive target cells to a greater degree than do non-CRISPR CAR-modified T cells (p<0.05 at all effector:target ratios of 2:1, 1:1 and 1:5). We, and others, have shown a decrease in cell surface CD5 protein expression following lentiviral gene transfer of a CD5-CAR transgene cassette in non-CD5 edited T cells using both VLR and scFv-based vectors. This downregulation has been speculated to be due to the interaction of the cell surface CAR with the target antigen. We also show that CD5-CAR expression in CD5-edited CAR-modified T cells is increased compared to CD5-CAR expression in non-CRISPR CAR-modified T cells (see Figure). Therefore, the interaction of the CAR with the cell surface antigen not only decreases antigen levels, but CAR levels as well. The decreased fratricide, increased CAR expression and activation of CD5-edited CAR-modified T cells in the presence of target cells should lead to more potent and durable anti-leukemia effects. Overall, our preclinical data show an advantage to using CD5-edited effector T cells when utilizing CAR-T cell therapy to target difficult to treat relapsed T-cell malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal